For babies exposed to opioids in the womb, parents may be the best medicine
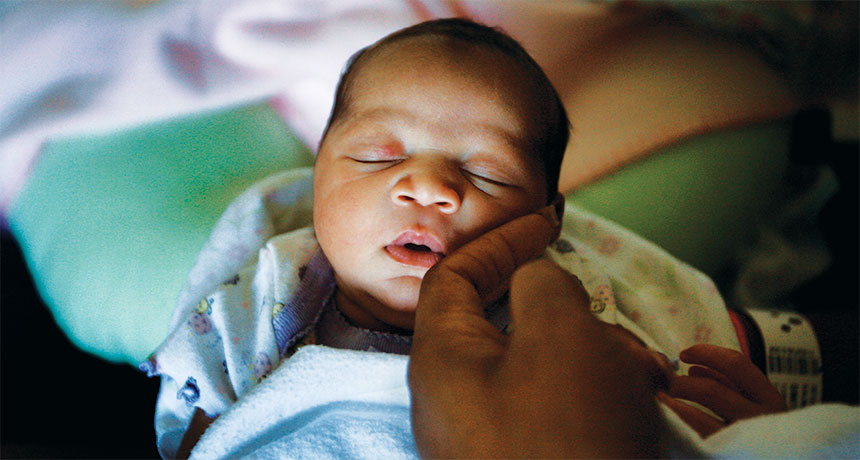
The first thing you’ll notice is the noise. Monitors beep steadily, relentlessly, ready to sound a car-alarm blare if a baby is in trouble.
The air has an astringent odor — not clean exactly, but reminiscent of an operating room (there’s one next door). Ceiling lights shine fluorescent white. Half are off, but glare from the monitors throws out extra light. It’s midday on a Friday, but it’ll be just as bright at midnight.
Here on the fourth floor of Yale New Haven Children’s Hospital, 10 tiny beds hold 10 tiny infants, each with Band-Aid–like patches stuck to their bodies to continuously monitor health. Between beds, nurses squeeze through narrow aisles crammed with folding chairs and plastic incubators. This space, one of five in the hospital’s neonatal intensive care unit, has the people and equipment needed to keep sick babies alive — heart rate monitors, oxygen tanks, IV poles to deliver medications.
Until recently, Yale’s NICU and hundreds like it across the country were considered the place to be for newborns withdrawing from opioid drugs. But now, as the number of drug-dependent babies surges, doctors here and elsewhere are searching for better options.
“We’re really focused on trying to get these kids out of the NICU,” says Yale pediatrician Matthew Grossman. “We’re looking at moms and the dads as the first line of treatment.”
The nationwide rate of babies withdrawing from opioids has soared — up nearly 400 percent from 2000 to 2012. The booming numbers are the bleak by-product of the United States’ ongoing battle with the drugs: Sales of prescription opioid pain relievers alone quadrupled from 1999 to 2010, and overdose deaths tripled from 2000 to 2014.
When pregnant women use opioids, the drugs pass from bloodstream to baby. After exposure in the womb to Vicodin, methadone or heroin, for example, babies can become dependent. At birth, when the drug flow stops, babies can go through agonizing withdrawal — body shakes, intestinal problems, constant crying. The condition is known as neonatal abstinence syndrome, or NAS.
But there’s no clear consensus on how to care for these struggling babies, Grossman says. Usually they’re whisked off to the NICU and treated with opioids. The drugs ease symptoms, but they prolong exposure to “really powerful and potentially dangerous medications,” he says.
At Yale, NAS babies used to spend weeks in the NICU — they still do in many U.S. hospitals. But in the last few years, Grossman and others have begun to question this method of care. Infants suffering from opioid withdrawal might actually do better back in parents’ arms, away from the high-tech hubbub. Comfort is key. Quiet, dark environments, swaddling, breastfeeding, rocking and holding, no unnecessary tests — it’s baby care 101.
That’s hard to do in the busy, loud NICU, says Grossman. Plus, there’s no place for parents to stay. They can visit, perched on folding chairs wedged between beds, says Yale pediatrician Rachel Osborn, but moms and dads “often feel like they’re extraneous and in the way.”
Faced with these and other obstacles, Grossman, Osborn and others are radically redefining their methods. They’re examining traditional practices, testing new ideas and getting back to basics. The results have been dramatic.
“We’re treating the families with respect and the babies like babies,” Grossman says. The parents have everything the baby needs, he says. “It’s not a whole lot more complicated than that.”
State of alarm
Maine, Vermont and West Virginia reported the highest rates of neonatal abstinence syndrome, opioid withdrawal linked to maternal drug use. Maine, Maryland, Massachusetts and Rhode Island rates are from 2012; the rest are 2013.
Click or tap the map below to learn more.
Tough going
Families today are much more likely to deal with opioid use — and its consequences for newborns — than they were a decade ago.
In 2004, NAS rates were consistently low across the country: For every 1,000 babies born, roughly one was diagnosed with NAS. By 2013, when pediatrician Nicole Villapiano and colleagues examined rural versus urban data, rates were up across the board. But rural areas had been hit the hardest, with nearly 8 per 1,000 babies diagnosed with NAS, the researchers reported in February in JAMA Pediatrics.
In an urban hospital near Rhode Island’s Providence River, Villapiano witnessed infant opioid withdrawal firsthand. She was first assigned to the newborn nursery service at Women and Infants Hospital in Providence in 2011. “I imagined it’d be a joyful time, seeing babies going off with their families, having wonderful lives.”
Reality quickly dashed that hopeful picture. On any given day, she might see several babies at a time struggling with withdrawal. “These children were miserable,” says Villapiano, now at the University of Michigan in Ann Arbor. “Their cries were persistent and their irritability was profound.”
NAS isn’t easy to define. Babies suffer a wide range of symptoms. They’re sweating, shaking, stiff. Stools are loose, eating and sleeping are difficult, and crankiness is common. Babies with NAS can also have breathing problems, seizures and low birth weights.
The syndrome was first described in heroin-exposed infants. Scientists now know that all sorts of opioids used during pregnancy can trigger the condition, including “maintenance” drugs like methadone or buprenorphine used to treat opioid addiction, and even painkillers commonly prescribed during pregnancy, such as codeine and hydrocodone.
Not all infants exposed to opioids in utero go through withdrawal — and exactly what conditions lead to NAS is still unclear. The particular opioid and how much a pregnant woman uses, whether she takes certain antidepressants and even the number of cigarettes she smokes per day all seem to factor in, Stephen Patrick of Vanderbilt University in Nashville and colleagues reported in Pediatrics in 2015. A nonsmoking woman on oxycodone for a few weeks, for example, might have roughly a 1 percent chance of delivering a baby with NAS. For a pack-a-day smoker on antidepressants and buprenorphine for six months, the risk could be more than 30 percent.
Because opioids are such a broad family of addictive drugs, opioid-using moms don’t fit neatly into one category, says Ju Lee Oei, a neonatologist at the University of New South Wales in Sydney. “We need to be aware that Mrs. Smith down the road who’s getting a bit of codeine for her back pain could have a baby with NAS,” Oei says.
Some women give birth to NAS babies while recovering from opioid addiction — even though they’re doing everything doctors advise, says pediatrician Alison Holmes of Children’s Hospital at Dartmouth-Hitchcock in Lebanon, N.H.
“Sometimes people think, ‘Oh, these mothers are such horrible addicts,’ ” Holmes says. But a lot of the time, “they’re staying on their methadone, they’re staying on their buprenorphine, they’re keeping symptoms under control — but their babies are still going to withdraw.”
No one knows exactly what opioid exposure does to fetal brains, or how these kids will fare in the future. Certain brain regions may not grow correctly, previous studies have suggested. Children can also have vision trouble and may develop behavior and attention problems. One long-term Australian study published in February linked a diagnosis of NAS with poor academic performance — all the way up to age 12 or 13.
Whether that’s caused by NAS is hard to say, says Oei, a coauthor of the study. Poverty, poor childhood nutrition and prenatal exposure to alcohol or other drugs could also come into play. But the results are a red flag for all those newly diagnosed babies. “You expect your baby to go to school and get good grades,” Oei says. But from as early as third grade, “these kids don’t seem to be able to do that.”
Still, research on NAS outcomes and potential treatments remains full of gaps, a 2015 report from the U.S. Government Accountability Office found. And there’s no nationally accepted treatment protocol for NAS. “Everyone’s doing it their own way,” says Scott Wexelblatt, a pediatrician at Cincinnati Children’s Hospital Medical Center.
Time for a change
The traditional way to assess NAS was published more than 40 years ago by neonatologist Loretta Finnegan, now at the College on Problems of Drug Dependence in Philadelphia. Every four to eight hours, sometimes more frequently, nurses evaluate symptoms using a detailed scoring list: the Finnegan Neonatal Abstinence Scoring System. Hit a certain score, and doctors will start up the withdrawal-easing opioids, typically morphine or methadone.
But there’s a push and pull between managing withdrawal and dosing babies with more drugs, Wexelblatt says. “We don’t want to expose babies to opioids unless we really need to.”
Care of NAS babies varies widely in hospitals across the United States, according to a study in the May–June Academic Pediatrics. Some newborns may be getting too much opioids. To see if standardizing care could help infants get off the drugs faster, Wexelblatt and colleagues trained nurses on Finnegan scoring and outlined a detailed protocol for weaning.
That simple step made a big difference. Hospitals that adopted the protocol cut infant stays from an average of 31.6 days before the intervention to 23.7 days afterward, Wexelblatt’s team reported in 2015 in Pediatrics. Duration of opioid treatment dropped as well. By 2016, hospital stays were down to 20 days, he says.
Now, 54 hospitals — almost all delivery hospitals in Ohio — use the weaning protocol, Wexelblatt says. The team has since refined its methods, focusing on family support and nonmedication options for care, like swaddling and breastfeeding. And as of 2013, every delivering mom in the Cincinnati region gets urine-tested for opioids upon admission so that care can start early, if needed. Ohio’s strategy is paying off: Doctors are using fewer opioids to treat NAS babies and the infants are getting out of the hospital faster too, early results suggest.
Researchers at Yale and Dartmouth-Hitchcock have also taken a hard look at the hospitals’ methods, starting with the Finnegan scoring system. Some aspects just didn’t make sense, Holmes and colleagues reported last June in Pediatrics. Nurses sometimes woke sleeping babies or removed them from family members’ arms for scoring, and they gave hungry babies points for crying.
“We said, ‘This is crazy,’ ” Holmes remembers. It makes more sense to just score the babies after they eat and while they’re being held. That way, she says, nurses might be able to sift the actual signs of withdrawal from the normal whines and wails of a hungry or tired baby.
Grossman and colleagues at Yale were skeptical too. Finnegan’s system looks for warning signs like vomiting and fever, but also gives points for sneezing and yawning. The final score guides doctors’ decision to dial meds up or down. “Is it truly best to give morphine to an infant who yawned 4 times instead of 3, as the [scoring system] guides us to do?” they asked in a Hospital Pediatrics commentary in February.
Grossman scoured the scientific literature, searching for clues to improve treatment. But research results bounced all over the place. “We ended up questioning everything,” he says. “It turned out there wasn’t really a good answer for anything we were doing.”
Family first
Around the same time Grossman was digging into research on opioid withdrawal in newborns, he had his first child, who screamed constantly. “I’m pacing in the middle of the night, thinking, if this was an NAS baby, he’d be on medication
immediately.”
Instead, Grossman paced and rocked and held his son — all of the tricks parents use to soothe a cranky newborn. As he found ways to settle the baby, he thought, what if NAS babies needed something similar?
The idea jibed with his experiences at the hospital. Sometimes withdrawing infants would do great for days—their moms were there, and Finnegan scores stayed low. But if moms had to leave, babies would backslide, and scores would rocket up again. “Do these kids need more mom or more meds?” Grossman and colleagues wondered. “We started to think, ‘Well, maybe it’s more mom.’ ”
At Dartmouth-Hitchcock, Holmes and her crew were coming up with their own ideas. The team stopped interpreting Finnegan scores so rigidly, for one. But their biggest change was keeping mothers and babies together, 24-7. It’s called “rooming-in,” and previous studies in Canada and other countries had suggested it might ease babies’ transition from the womb to the world.
“What withdrawing babies need is a calm, quiet, dark place where they can be held by a caring individual,” Holmes says. Her team focused on involving moms and families (and even volunteer cuddlers), and the hunch paid off. From 2012 to 2015, the average length of stay for morphine-treated NAS babies dropped from 16.9 days to 12.3 days. The fraction of babies given morphine plummeted too, from 46 percent to 27 percent. Now, two years later, that number has fallen even further — to just 20 percent, she says.
Holmes says her own kids joke about her work: “Babies like their mothers—surprise, surprise! What a discovery!” She laughs, and then adds, “They’re kind of right.”
Grossman’s team at Yale has pushed the family-focused approach even further. “Our mind-set is rooming-in on steroids,” he says. For NAS, parental care is considered more important—and more effective—than medication. Doctors ask parents: “How do we get you here or dad here or grandma here?” Grossman says. “Because that’s what your baby needs.”
His team rolled in other ideas too, like fortifying formula and pumped breast milk with extra calories. And hospital personnel stopped using Finnegan scores to guide medication dosing. Today, they base assessments on three simple parameters: whether an infant can eat, sleep and be consoled.
The patient rooms where parents can bunk with their babies are a world apart from the NICU. One room at Yale has a couch that converts into a bed and ceiling tiles with pictures of Elmo and Tweety Bird. Monitors are muted, nothing beeps incessantly and natural light pours in from the window. There’s plenty of space for parents to walk around and tend to their baby. In these rooms, “it feels like the parent is a necessary part of the care team,” Yale’s Osborn says.
In 2016, babies with NAS stayed in the Yale hospital just 5.9 days — a cliff dive compared with the 2008–2010 average of 22.4 days, Grossman, Osborn and colleagues reported online May 18 in Pediatrics. Even more staggering is the fraction of these babies treated with morphine: just 14 percent in 2016, down from 98 percent in 2008–2010.
Taking the plunge
From 2008 to 2016, the proportion of opioid-withdrawing infants treated with morphine at Yale New Haven Children’s Hospital dropped from 98 percent to 14 percent, a drastic reduction in the number of babies given the medication.
Click or tap the graph below to learn more.
Yale’s approach basically comes down to common sense, Grossman says: a quiet room, lots of holding, feeding when hungry and simply keeping babies with mom and dad. “It’s not rocket science,” he says. Medication became more of a plan B.
Still, other doctors looking to transform NAS treatment may run into barriers. Not all U.S. hospitals are set up like Dartmouth-Hitchcock or Yale New Haven, Wexelblatt says. There’s not always room for mom to stay with her baby once she’s released. And universal drug testing of moms won’t work everywhere, he warns. In Tennessee, a law passed in 2014 allowed new mothers to be prosecuted for using illegal drugs while pregnant if the newborn was harmed. The law expired last July, but such legislation drives women away from medical care, Wexelblatt says.
It could be that the best care for babies begins with care and compassion for moms. Rather than blame mothers, Holmes says, “We need to do as much as we can to support them in being good parents.”